Extract from "General Relativity"
De Laplace
pp 80-83
1 Thermodynamics
Thermodynamical systems can be extraordinarily complicated; for example, a great number of processes can be going on in a star simultaneously. We want to try to explain the basic general ideas, restricting ourselves to the simplest systems.
During thermodynamical processes certain elements of matter, with their properties, remain conserved, for example, in non-relativistic thermodynamics molecules or atoms and their masses. In the course of transformations in star or during nuclear processes the baryons with their rest mass are conserved instead. We shall therefore relate all quantities to these baryons. If, for example, we choose a volume element of the system, then we shall take as four-velocity ui of this element the average baryon velocity. The flow (motion) of the system will therefore be characterized by a four-velocity field
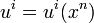 ,
, 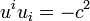 (8.65)
(8.65)To set up the basic thermodynamical equations one first go to the local rest system
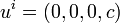 (8.66)
(8.66)of the volume element under consideration and regard this volume element as a system existing in equilibrium (of course it interacts with its surroundings, so that the whole system is not necessarily in equilibrium); that is one introduces for this volume element the fundamental thermodynamic state variables, for example,
-
 baryon number density,
baryon number density,
-
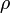 baryon mass density,
baryon mass density,
-
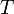 temperature,
temperature,
-
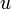 internal energy per unit mass,
internal energy per unit mass,
-
 entropy per baryon mass,
entropy per baryon mass,
-
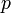 isotropic pressure,
isotropic pressure,
-
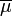 chemical potential,
chemical potential,
-
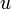 free energy per unit mass.
free energy per unit mass.
"Density" here always means "per three-dimensional volume in the local rest system"; the entropy density, for example, would be given by sρ. There exist relationships between state variables which in the simplest case express the fact that only two of them are really independent, and from, knowledge of the entropy as a function of the energy and the density, or of the specific volume v = 1 / ρ,
 (8.68)
(8.68)one can calculate the other quantities, for example,
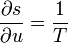
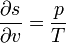
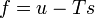 etc. (8.69)
etc. (8.69)For the interaction of the volume element we have balance equations. These are the law of conservation of baryon number
 (8.70)
(8.70)(generalized mass conservation), the balance equation for energy and momentum, formulated as the vanishing of the divergence of the energy-momentum tensor 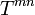 ,
,
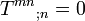 (8.71)
(8.71)(generalized first main law), and the balance equation for the entropy
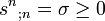 (8.72)
(8.72)wich says that the density of entropy production is always positive or zero (generalized second law od thermodynamics). Of course these equations take on a physical meaning only if the entropy current density sn and the energy momentum tensor Tmn are tied up with one another and with the thermodynamic quantities (8.67).
This can be done as follows. One uses the projection tensor
 , (8.73)
, (8.73)to decompose the energy momentum tensor into components parallel and perpendicular to the four-velocity
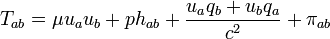
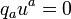
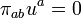
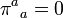 (8.74)
(8.74)and links the quantities which then occur to the thermodynamic state variables and to the entropy current vector. The coefficient of hab is the isotropic pressure, the internal energy per unit mass u is coupled to the mass density μ in the rest system of the matter by
 (8.75)
(8.75)and the heat current qi (momentum carried density in the rest system) goes into the entropy current
 (8.76)
(8.76)Equation (8,76) says that the entropy flows in such a way that it is carried along convectively with the mass (first term) or transported by the flow of heat (generalization of dS = dQ / T).
We now want to obtain an explicit expression for the entropy production density σ. Upon using (8.70) and the equation
![s_{,n}u^n = \frac{1}{T}\left(\frac{\mu c^2}{\rho}\right)_{,n}u^n + \frac{p}{T}\left(\frac{1}{\rho}\right)_{,n}u^n = \frac{1}{\rho T}\left[(p+\mu c^2)u^i{}_{;i}+\mu_{,n}u^n c^2\right]](/wiki/images/math/9/c/a/9ca43881ca3a5b680d1a43367925eb57.png) (8.77)
(8.77)which follows from (8.68), (8.69) and (8.75), we obtain
![\sigma = s^n_{;n}=\frac{1}{T}\left[(p+\mu c^2)u^i_{;i}+\mu_{,n}u^n c^2\right]+\left(\frac{q^n}{T}\right)_{;n}](/wiki/images/math/c/b/e/cbece6aa95fea846cc99dae59f488a54.png) } (8.78)
} (8.78)Since the terms in the square brackets can be written in the form
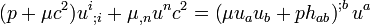 (8.79)
(8.79)and the divergence of the energy momentum tensor vanishes, (8.78) implies the relation
![\sigma=[T^{mn}-\mu u^m u^n - p h^{mn}]_{;m}\frac{u_n}{T}+\left(\frac{q^n}{T}\right)_{;n}](/wiki/images/math/6/c/4/6c400821ef8570a352edab0f55b23745.png) (8.80)
(8.80)which, bearing in mind the definition (8.74) of qn and using (5.32), can be cast finally into the form
 (8.81)
(8.81)In irreversible thermodynamics one can satisfy the requirement that 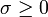 in many cases by writing the right-hand side of (8.81) as a positive-definite quadratic form, that is, by making an assumption of linear phenomenological equations. For the particular case πmn = 0, when (8.81) reduces, because of (8.74), to
in many cases by writing the right-hand side of (8.81) as a positive-definite quadratic form, that is, by making an assumption of linear phenomenological equations. For the particular case πmn = 0, when (8.81) reduces, because of (8.74), to
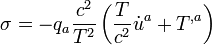
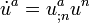 (8.82)
(8.82)this ansatz means that
 (8.83)
(8.83)which represents the relativistic generalization of the linear relation between heat current and temperature gradient.
In many cases one can ignore irreversible processes. If the system is determined by only two state quantities in the sense of (8.68), this means because of (8.81) that complete, exact reversibility (σ = 0) is possible either only for certain metrics (whose Lie derivatives vanish), or for especially simple media, whose energy-momentum tensor has the forma
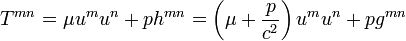 (8.84)
(8.84)Such a medium is called a perfect fluid or, for p=0, dust.
2 Equation (5.32) (Lie derivative)
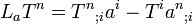
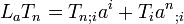
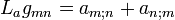 (5.32)
(5.32)




